INTRODUCTION
The dental pulp is a unique connective tissue containing fibers , cells, extracellular matrix, and a wide nerve and vascular plexus. Dental pulp fibroblasts (DPFs) exhibit some singularities with respect to fibroblasts present in other connective tissues, such as the expression of tenascin, osteonectin, and tissue-related extracellular matrix (ECM) proteins.
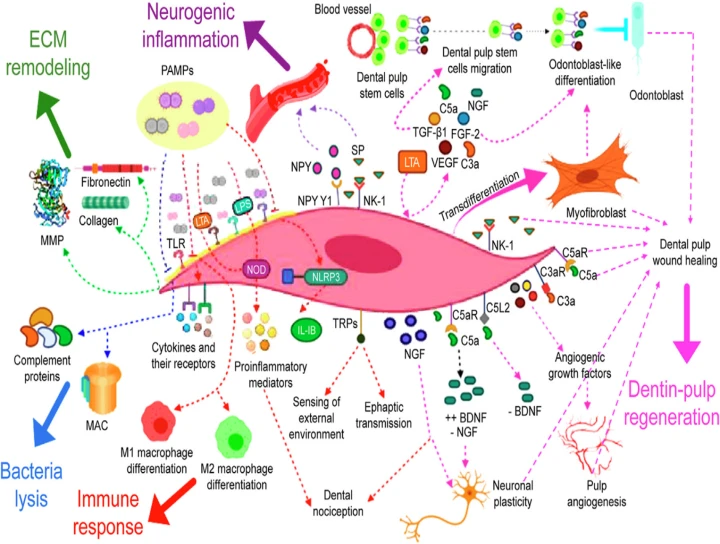
Dental pulp fibroblasts (DPFs) participate in extracellular matrix (ECM) remodeling through the synthesis of collagen and fibronectin and its degradation by matrix metalloproteinases (MMPs). In response to bacterial stimulation, DPFs express all complement proteins, including the membrane attack complex (MAC), allowing the lysis of cariogenic bacteria. Furthermore, these cells have the ability to secrete and respond to various cytokines.
DPFs also express the NLRP3/caspase-1 inflammasome, generating the release of interleukin (IL)-b, the most potent proinflammatory cytokine. They express tolllike (TLR) and nucleotide-binding oligomerization domain (NOD) receptors, and thus recognize pathogen-associated molecular patterns (PAMPs) and regulate the expression of various proinflammatory mediators, through the recognition of PAMPs like lipoteichoic acid (LTA) and lipopolysaccharides (LPS).
In response to inflammatory mediators, DPFs secrete Substance P (SP) and express receptors for neuropeptides such as neurokinin-1 receptor (NK-1) and NPY Y1 receptor (NPY Y1), thus participating in neurogenic inflammation and dental pulp wound healing and amplifying the pulp immune response.
DPFs contribute to dental nociception, by secretion of neural growth factor (NGF) that sensitizes afferent nerve fibers and express transient receptor potential channels (TRP) that are sensors of external environment. TRP channels could allow DPFs to perform ephaptic transmission, although this is yet to be elucidated.
DPFs interact with macrophages and modulate their differentiation into M1 (proinflammatory) macrophages to control infection and M2 (anti-inflammatory) to start pulp healing, demonstrating the active participation of DPFs in local immune response and inflammation. DPFs also actively participate in dentin-pulp regeneration through the secretion of growth factors such as transforming growth factor beta-1 (TGF-b1), basic fibroblast growth factor (FGF-2), vascular endothelial growth factor (VEGF), complement proteins (C3a and C5a), and NGF, allowing dental pulp stem migration and odontoblast-like differentiation.
They also secrete angiogenic growth factors for pulp angiogenesis. DPFs secrete the brain-derived neurotrophic factor (BDNF) when C5a binds to their C5aR receptor, decreasing the expression of NGF, which increases in inflamed pulp tissue; BDNF decreases when C5a binds to the C5L2 receptor. Both, NGF and BDNF are essential for neuronal plasticity.
Finally, DPFs participate in dental pulp wound healing and dentin-pulp regeneration by transdifferentiating into myofibroblasts; these latter cells facilitate the reorganization of the ECM in injured pulp and can differentiate into odontoblast-like cells, with the capacity to synthesize reparative dentin.
SIGNIFICANCE
Dental pulp fibroblasts are more than mere passive cells in pulp biology, playing pivotal roles in immunoregulatory mechanisms, control of inflammation, and dentin-pulp regeneration. They could be an important milestone in regenerative endodontics also.
CONCLUSIONS AND FUTURE PERSPECTIVES
Overall, considering the pivotal role of DPFs in health and disease, as well as their potential therapeutic application in regenerative endodontics, it is clear that this cell type is not a mere bystander in the pulp-dentin complex. In the near future, molecular programs, proteomic profiling, and artificial intelligence, owing to their unique characteristics and performance, could help confirm known findings and unveil novel functions of DPFs, further establishing their status as star cells of the pulp tissue. These approaches could also be an important milestone in developing fibroblast-based therapies.


No Any Replies to “DENTAL PULP FIBROBLAST: A STAR CELL”
Leave a Reply